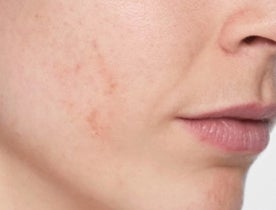
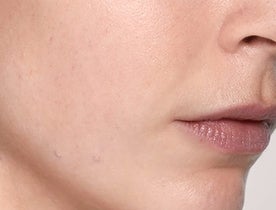
What does Restylane® SKINBOOSTERS™ do?
Restylane® SKINBOOSTERS™ is the only hyaluronic acid skin quality injectable that is clinically proven to provide 6 key skin quality benefits for healthy glowing skin that lasts up to 15 months.1-14*
Deep hydration for a refreshed glow
Deep hydration for refreshed and even skin that seems to glow from within.1
Skin surface evenness
Improve skin surface evenness by reducing the appearance of pores.4
Smooth texture
Reduce roughness and optimize texture to rebalance skin surface.2-6
Even skin tone
Reduce pigmentation and age spots for brighter skin.8
Reduced fine lines & wrinkles
Smooth out and correct fine lines and wrinkles, and improve elasticity.3,4,7
Reduced scarring
Reduce acne scarring in just 8 weeks with long-lasting results.1